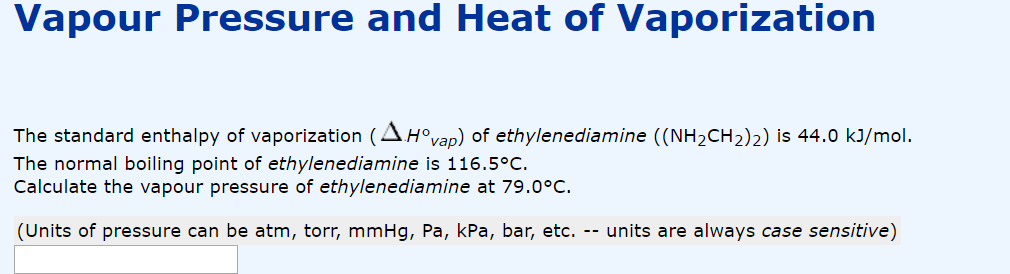
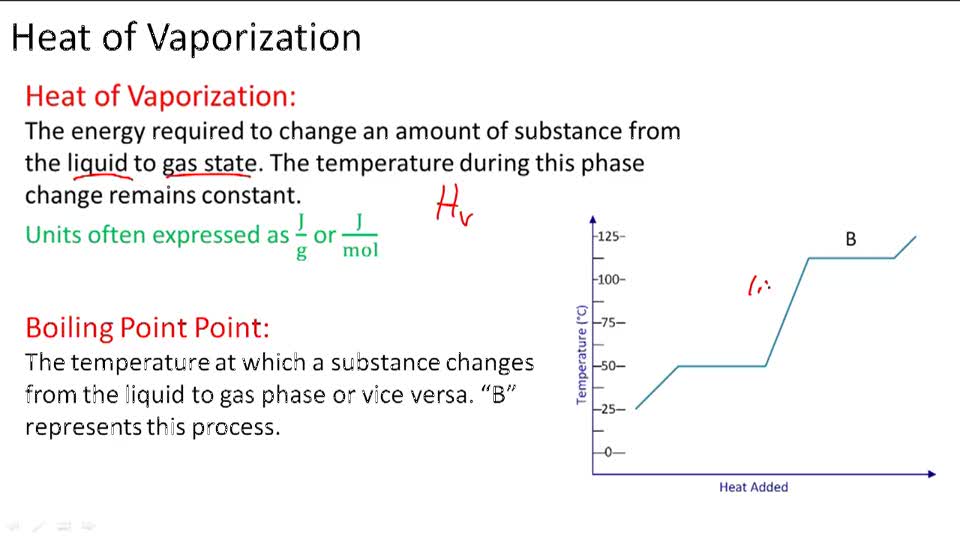
Standard vaporization enthalpy of benzene at its boiling point is 30.8 kJ mol^-1; for how long would - Sarthaks eConnect | Largest Online Education Community
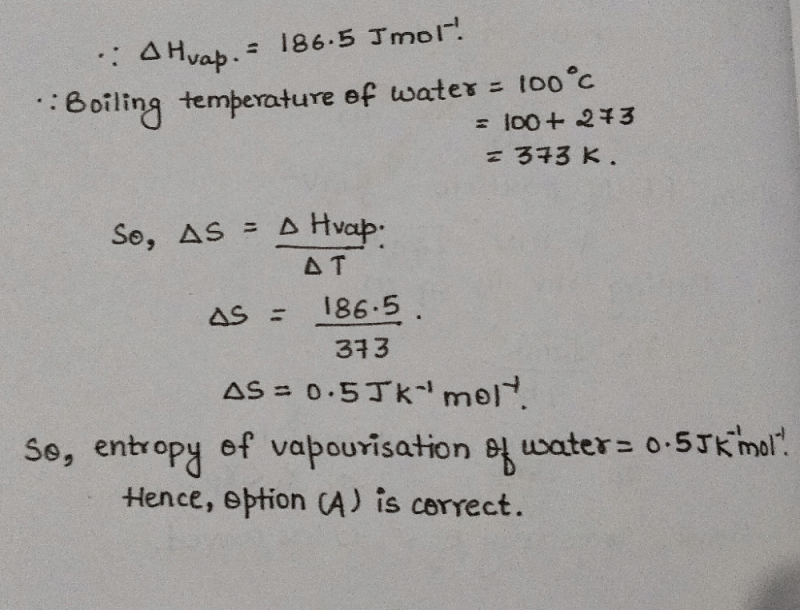
If the enthalpy of vaporization of water is 186.5 J the entropy of its vaporization will be a) b) c) d) Correct answer is option 'A'. Can you explain this answer? -

Vapor Pressure, Vaporization Enthalpy, Standard Enthalpy of Formation and Standard Entropy of n-Butyl Carbamate - ScienceDirect
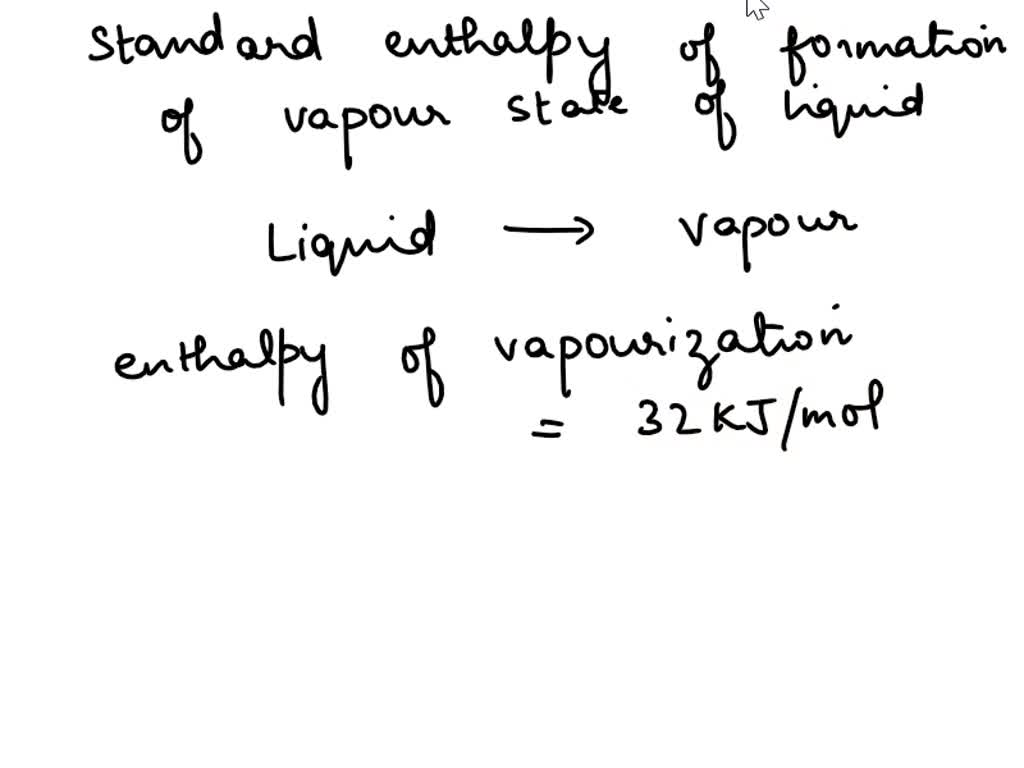
SOLVED: The value of enthalpy of vaporization of a liquid is 32 kJ/mol. The standard enthalpy of formation of that liquid is 20 kJ/mol. Calculate the standard enthalpy of formation of the
![standard enthalpy of vapourisation A HO) water 100°C is 40.66 kJmol.The internal energy of vapourisation of water 100°C (in kJmot) is [AIPMT (Prelims)-2012] (2) +40.66 (4) -43.76 funtor is 1 435 kcal/mol. (1) +43.76 (3) +37.56 standard enthalpy of vapourisation A HO) water 100°C is 40.66 kJmol.The internal energy of vapourisation of water 100°C (in kJmot) is [AIPMT (Prelims)-2012] (2) +40.66 (4) -43.76 funtor is 1 435 kcal/mol. (1) +43.76 (3) +37.56](https://toppr-doubts-media.s3.amazonaws.com/images/8783536/2a161f71-6f15-470c-af56-293d004f42a8.jpg)
standard enthalpy of vapourisation A HO) water 100°C is 40.66 kJmol.The internal energy of vapourisation of water 100°C (in kJmot) is [AIPMT (Prelims)-2012] (2) +40.66 (4) -43.76 funtor is 1 435 kcal/mol. (1) +43.76 (3) +37.56
Standard enthalpy of vaporisation ΔvapH° for water at 100°C is 40.66 kJ mol^-1. - Sarthaks eConnect | Largest Online Education Community

Estimating Enthalpy of Vaporization from Vapor Pressure Using Trouton's Rule | Environmental Science & Technology