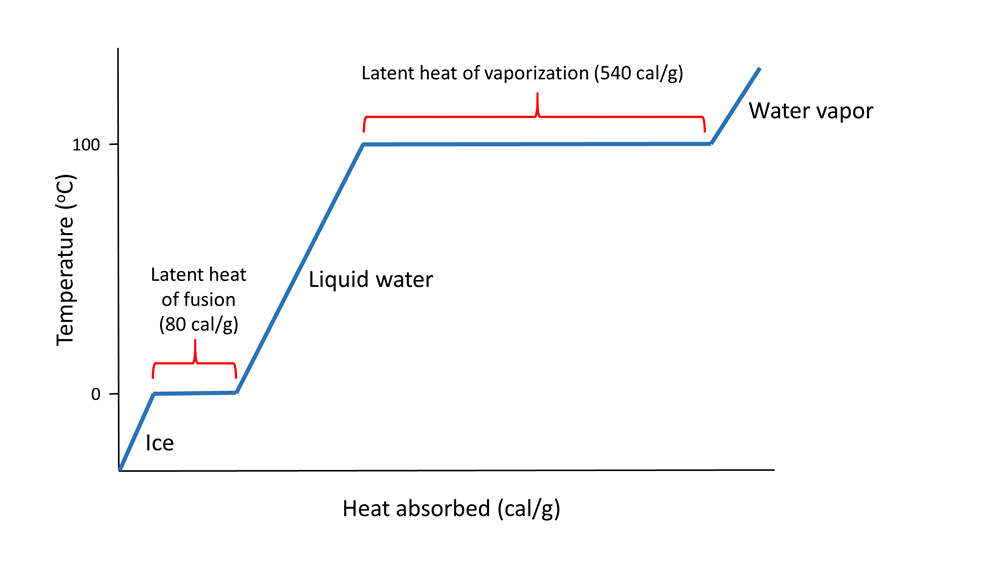
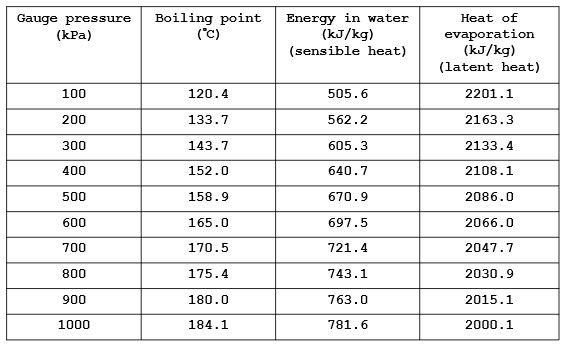
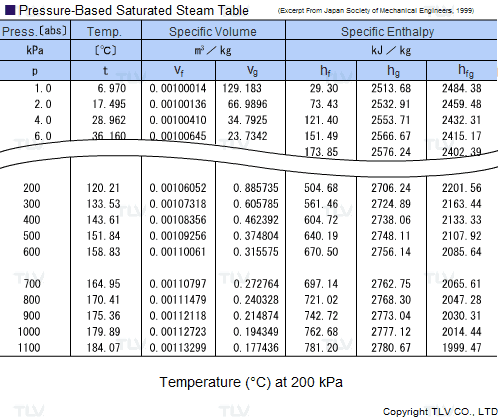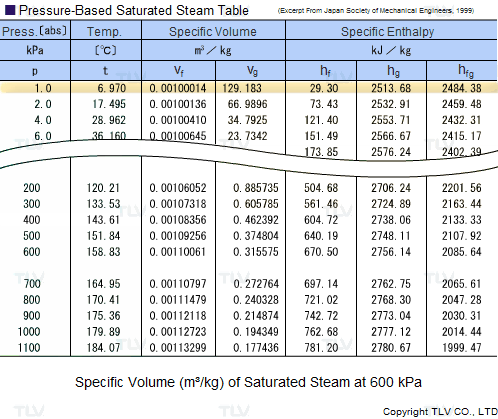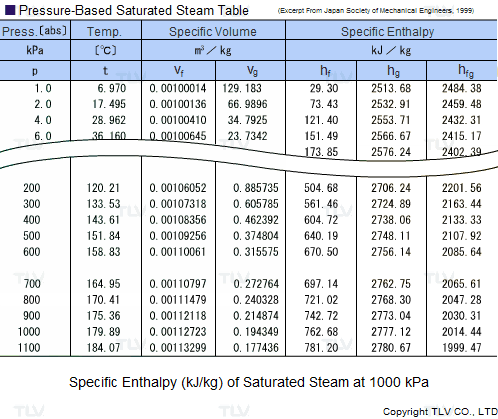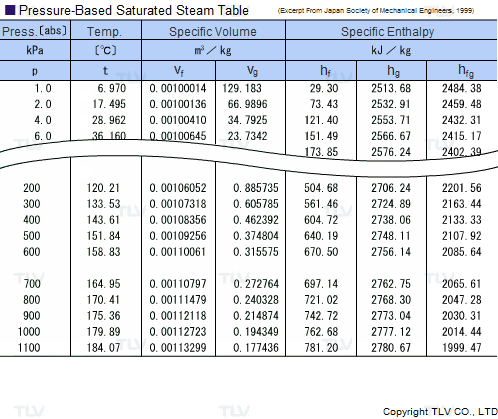
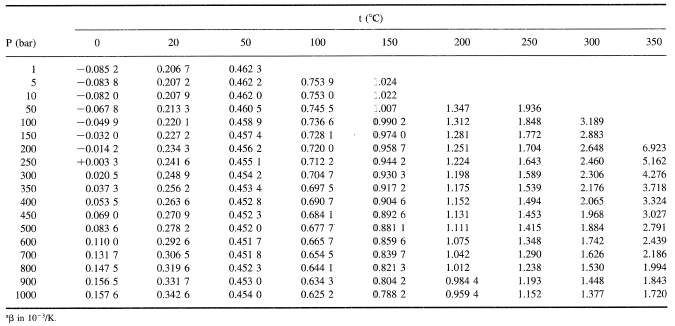
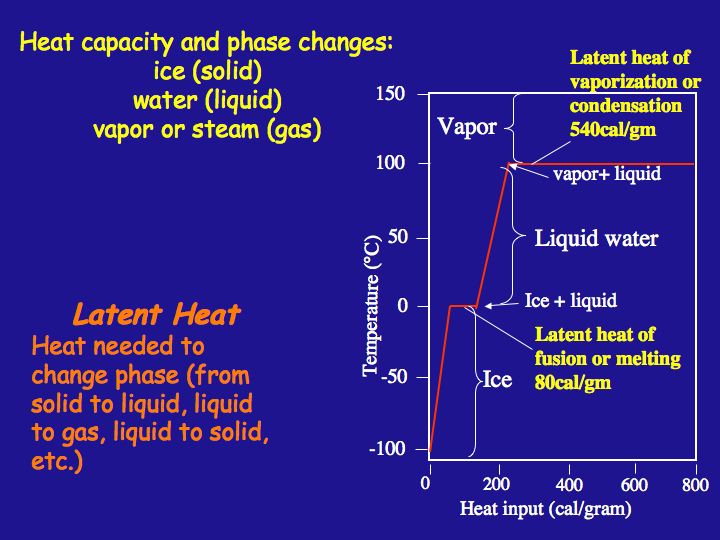
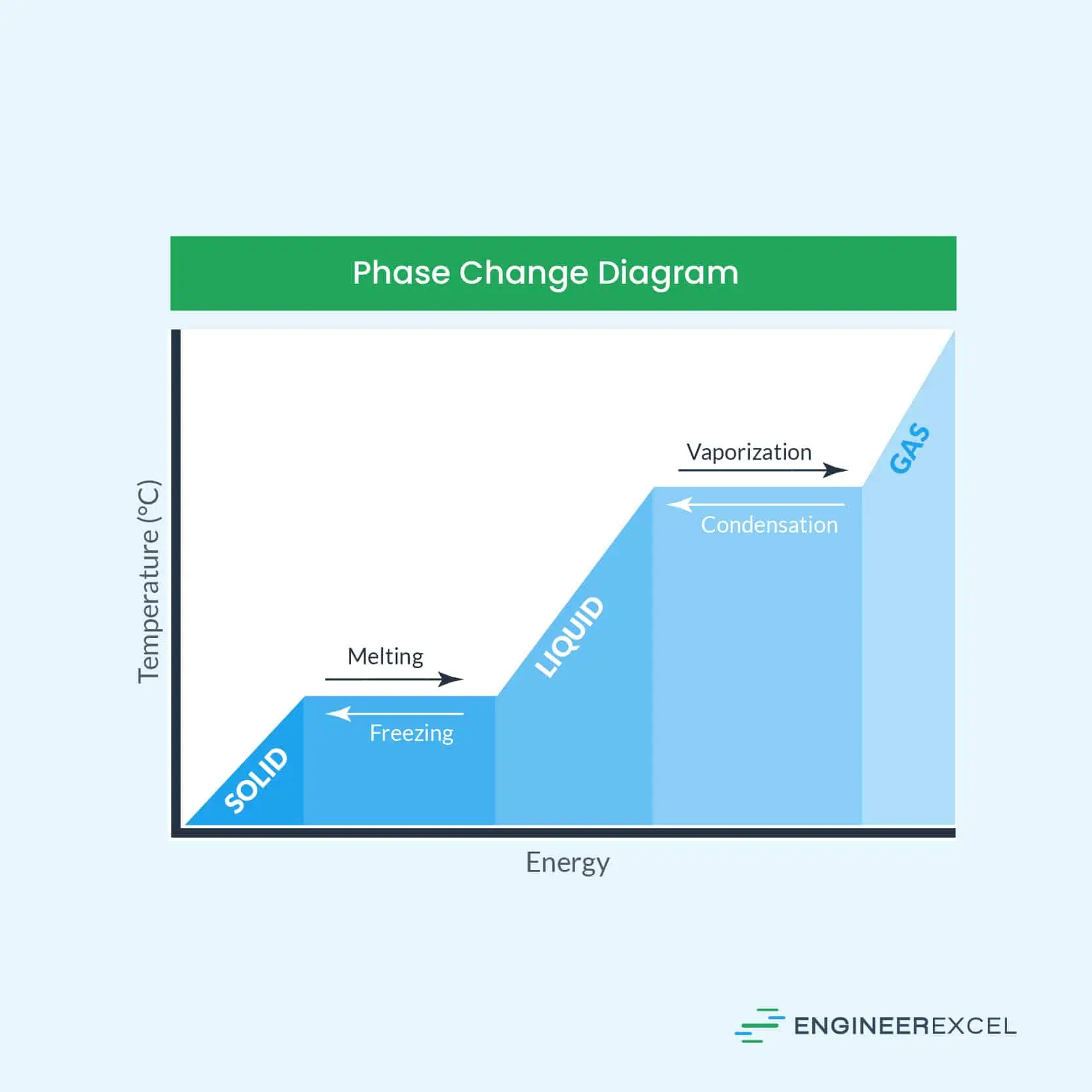
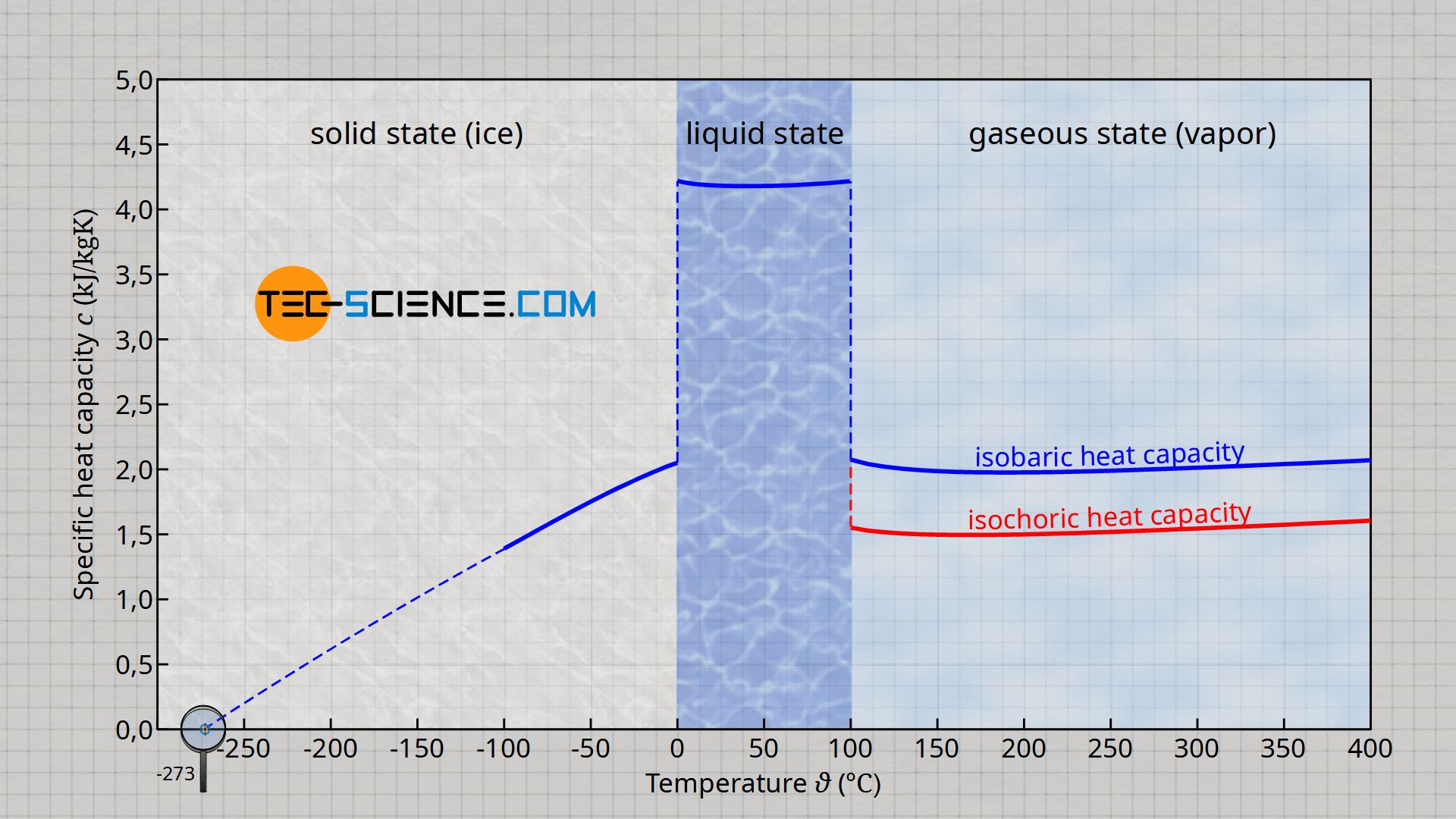
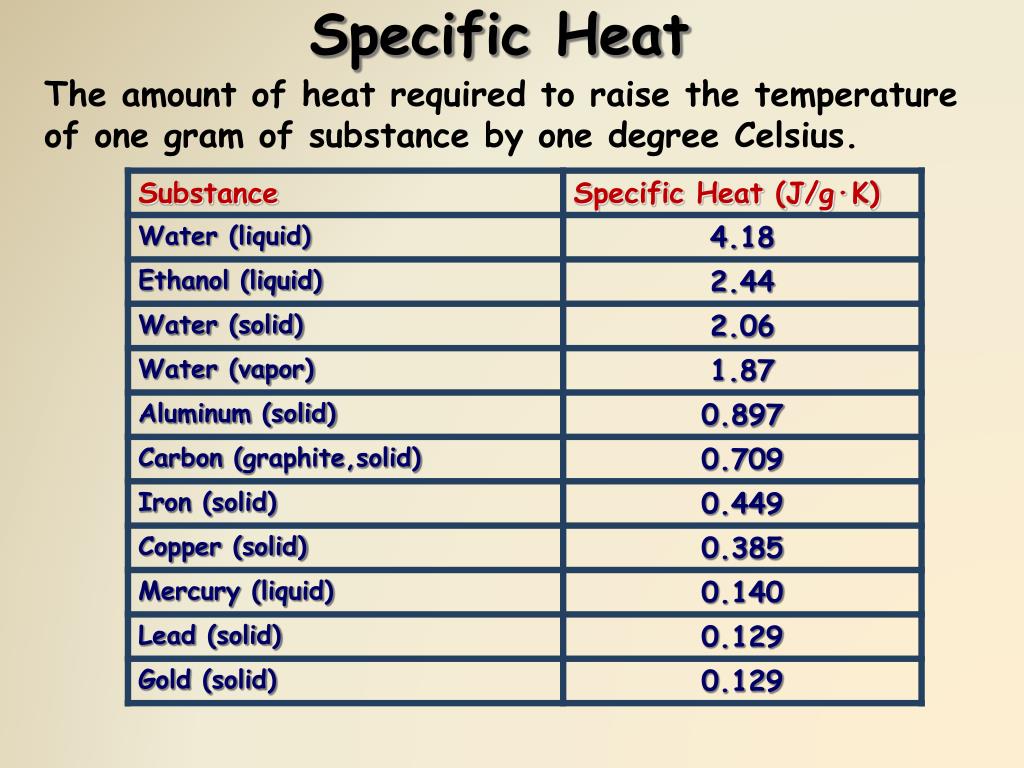
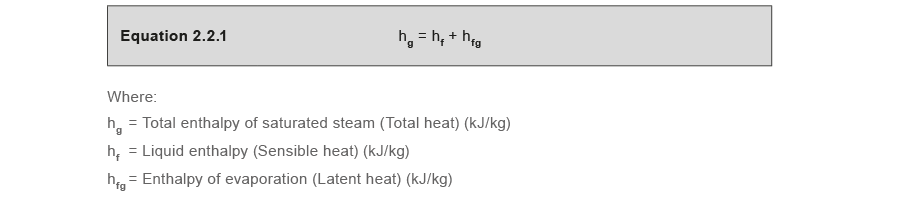The latent heat of vapourisation of water at 100 Celcius is 540 cal/g . Calculate the entropy increase when one mole of water at 100 Celcius is evaporated.
![PDF] Dependence of the isobaric specific heat capacity of water vapor on the pressure and temperature | Semantic Scholar PDF] Dependence of the isobaric specific heat capacity of water vapor on the pressure and temperature | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/6c137407717ac2381ee1b2a89743eedede34ab16/5-Figure5-1.png)
PDF] Dependence of the isobaric specific heat capacity of water vapor on the pressure and temperature | Semantic Scholar

SOLVED: How much energy (in kJ) is required to take 35 g of water vapor at 110°C to liquid water with a final temperature of 37°C? Enthalpy of fusion: 6.01 kJ/mol Enthalpy

Standard-state specific heat ratio of carbon dioxide and water vapor... | Download Scientific Diagram