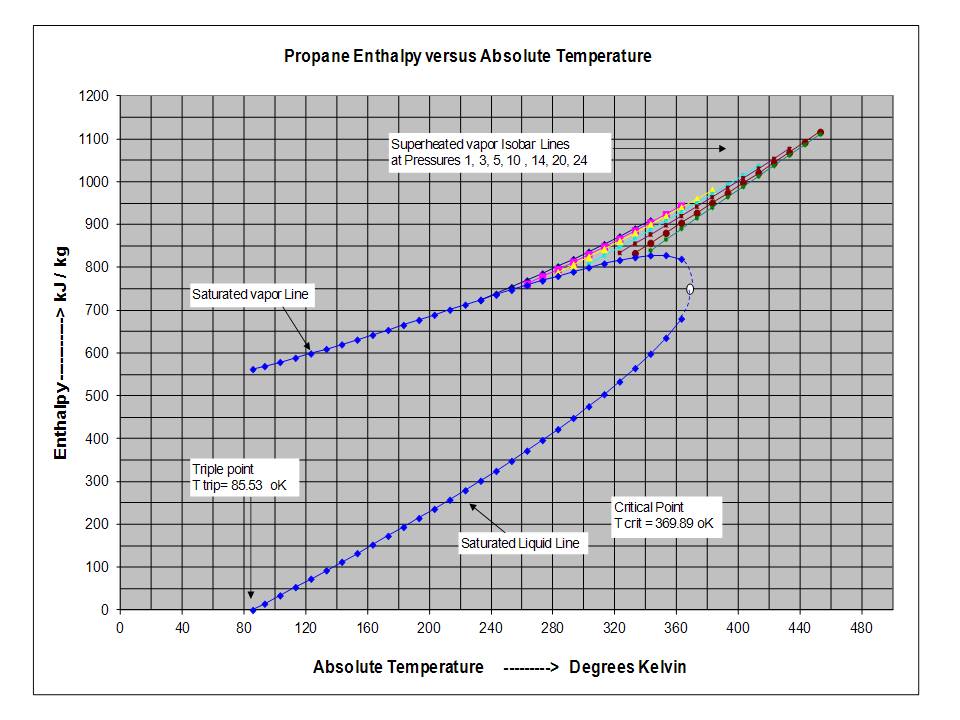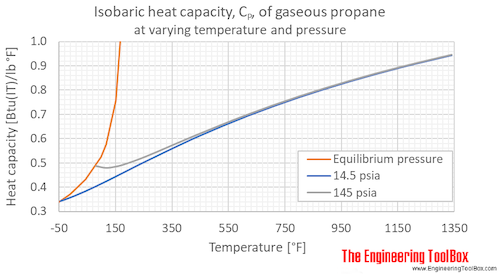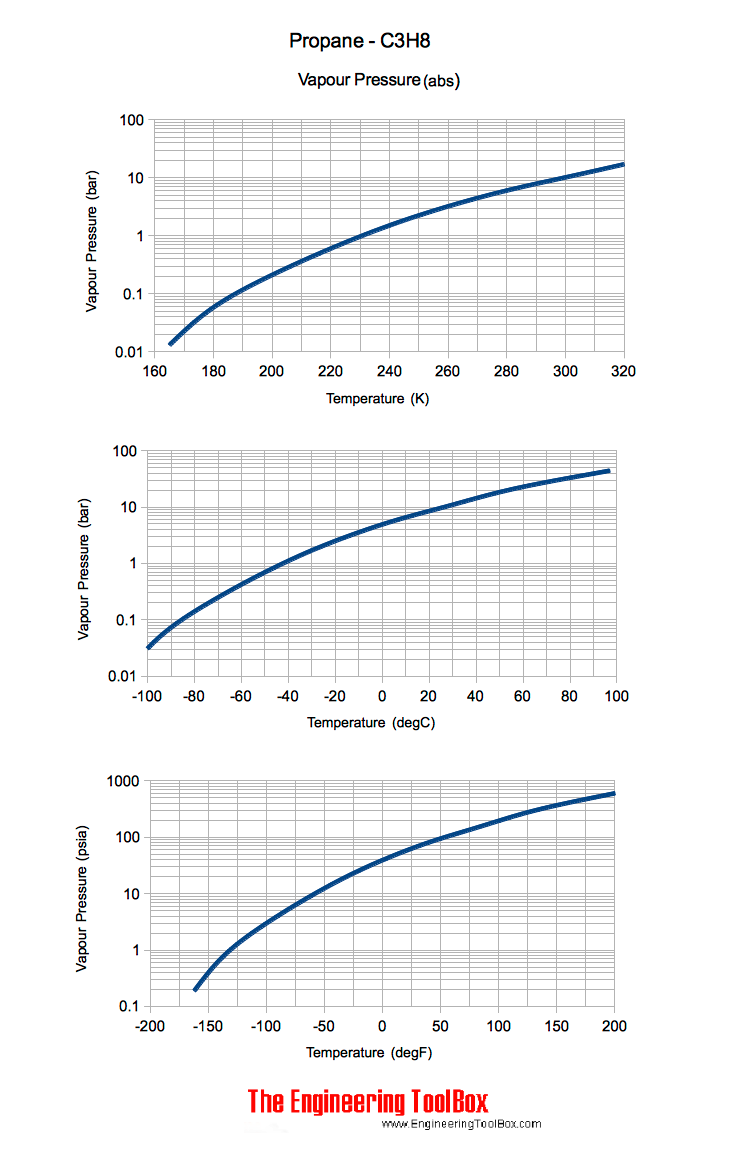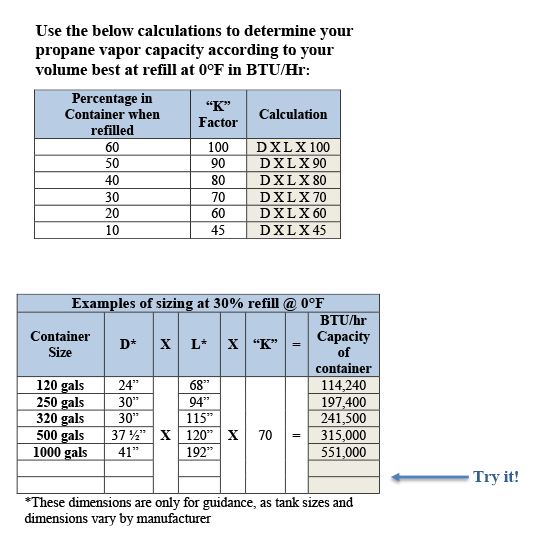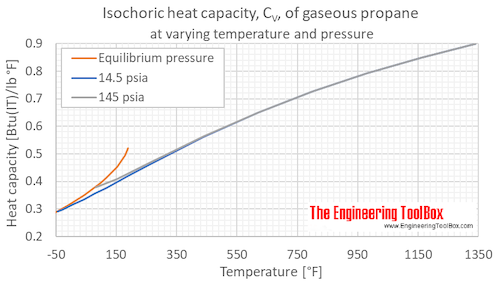
Table 3 from Thermodynamic Properties of Propane. II. Molar Heat Capacity at Constant Volume from (85 to 345) K with Pressures to 35 MPa | Semantic Scholar
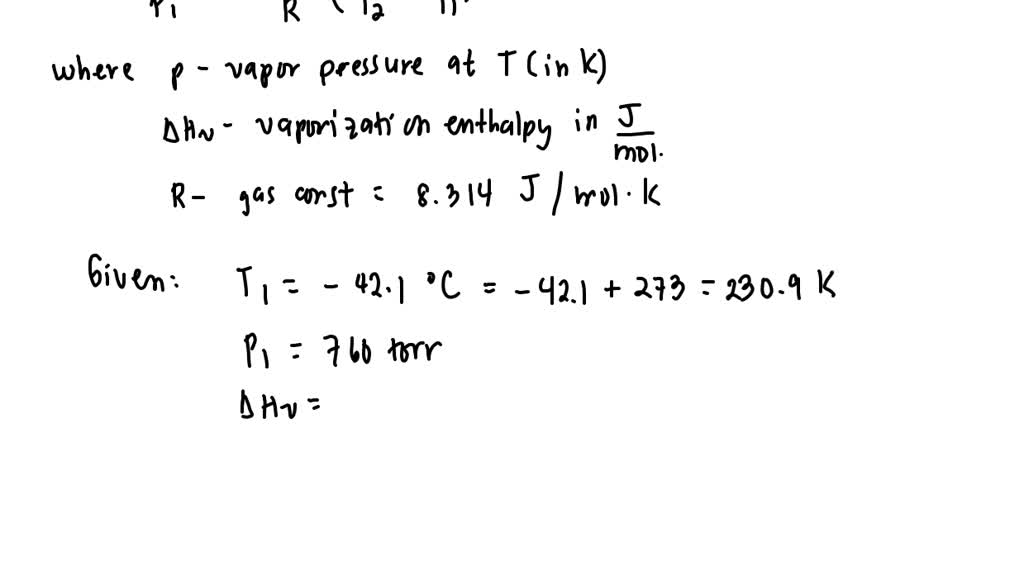
SOLVED: The enthalpy of vaporization of propane is 19.0 kJ/mol and its normal boiling point is -42.1 °C. Using the Clausius-Clapeyron equation, calculate the temperature at which propane has a vapor pressure

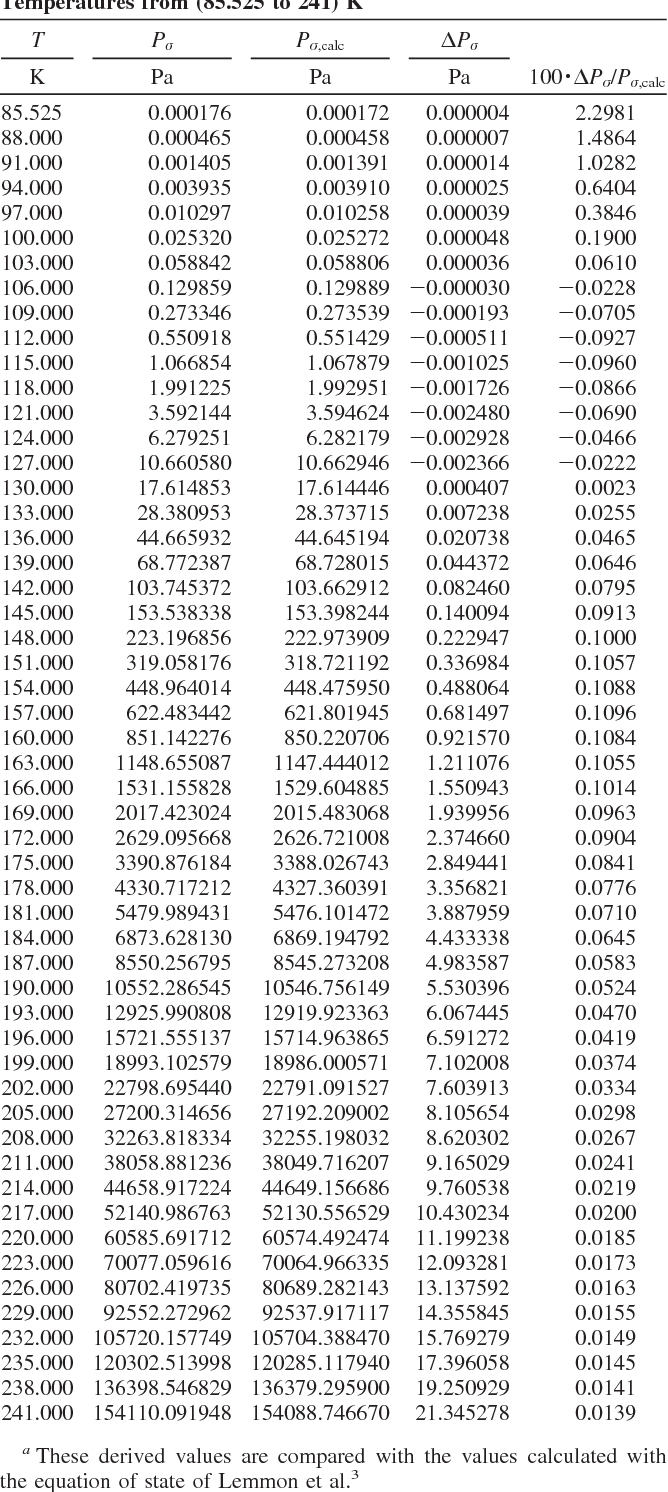
Table 2 from Thermodynamic Properties of Propane. II. Molar Heat Capacity at Constant Volume from (85 to 345) K with Pressures to 35 MPa | Semantic Scholar
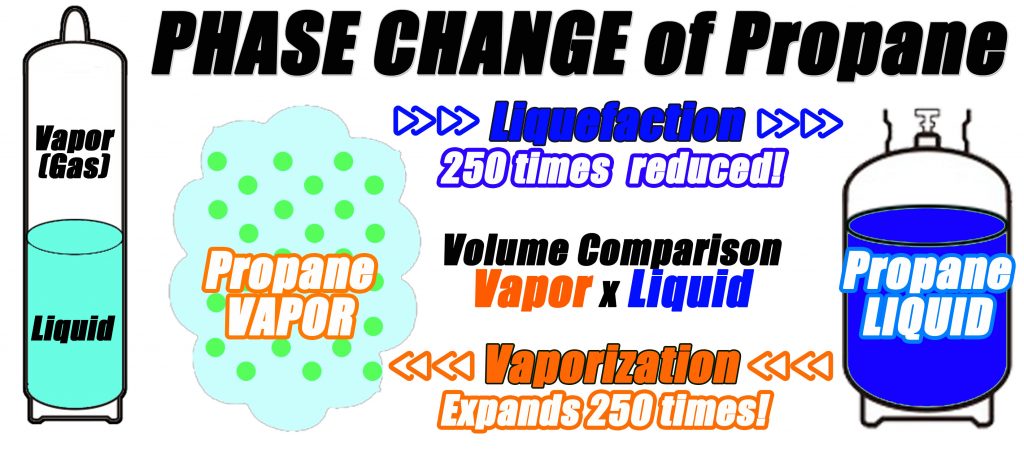
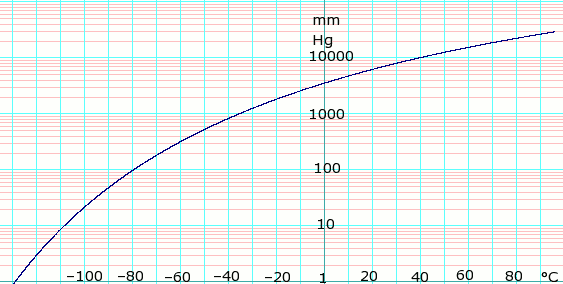
What's LPG? Why Vaporizer? – Useful Information for Users - KAGLA | Vaporizers for Propane-Butane (LPG), Ammonia and Synthetic Natural Gas

The standard heat of combustion of propane is -2220.1 kJ/mol. The standard heat of vaporization of liquid water is 44 kJ/mol. What is the triangle H^{o} of the reaction :C_{3} H_{8} (g)+
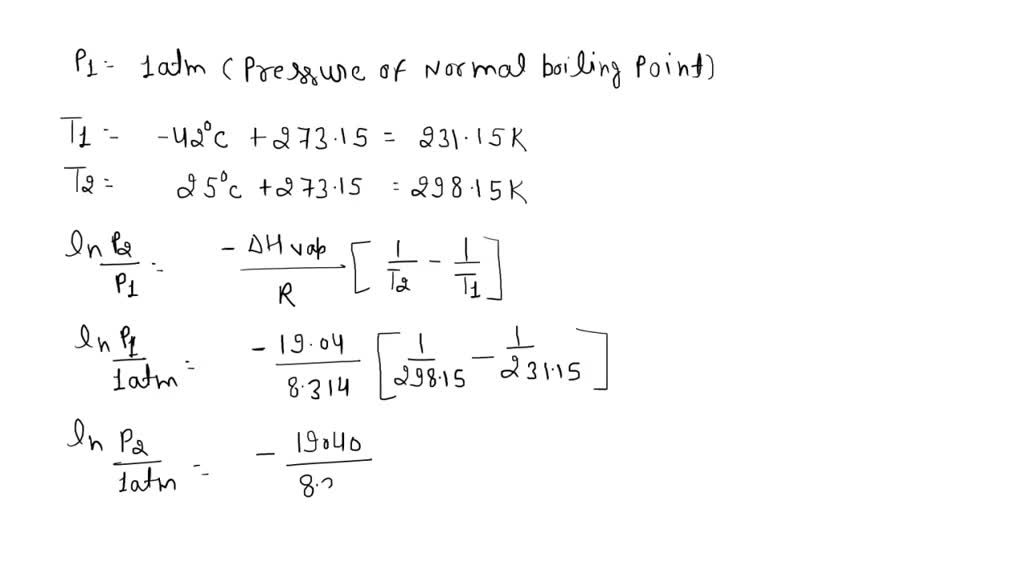
SOLVED: Propane has a normal boiling point of -42.0°C and a heat of vaporization of 19.04 kJ/mol. What is the vapor pressure, in torr, of propane at 25°C?

Approximated latent heat of vaporization for (a) butane; (b) isobutane;... | Download Scientific Diagram

OneClass: The heat of vaporization of propane (C3H8) is 19.4 kJ/mol. Calculate the change in entropy ...