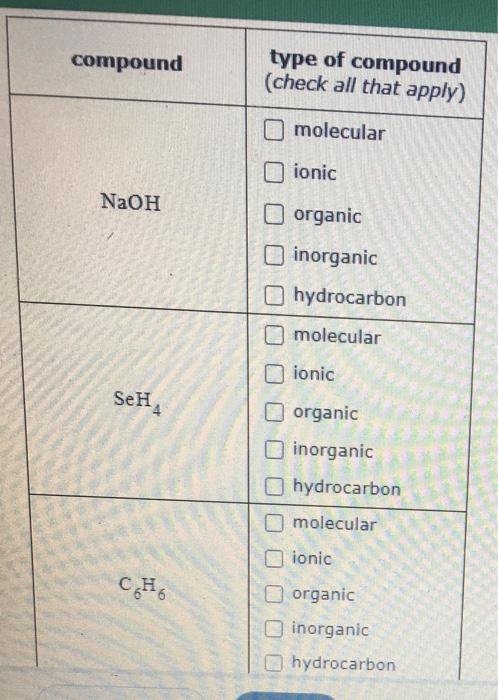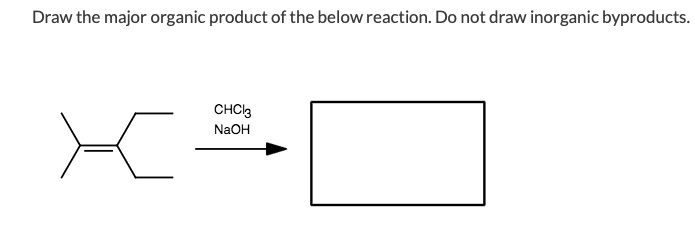
SOLVED: Draw the major organic product of the below reaction. Do not draw inorganic byproducts. CHCl3 + NaOH

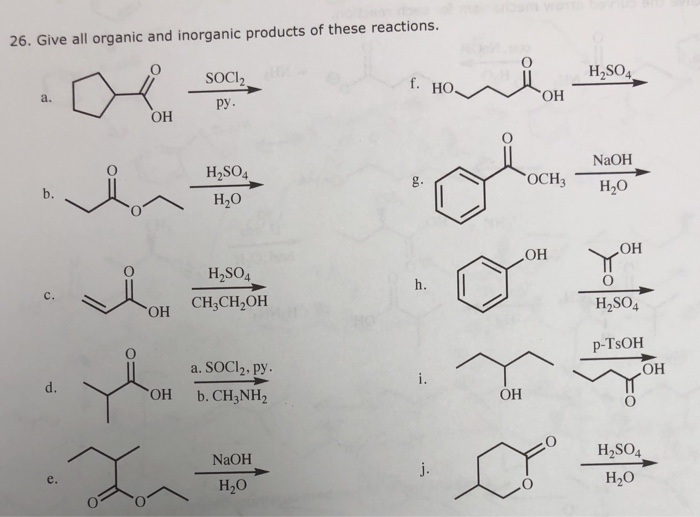
SOLVED: (siakBH HzOz NaOH HCrO4 SOCIz H2C-CHCH2OH) Work out the above synthesis on a separate sheet of paper; and then draw the organic product of the reaction. You do not have to

If is mixed with `NaOH` solution, acid-base reaction occurs and snatches from organic molecule. - YouTube

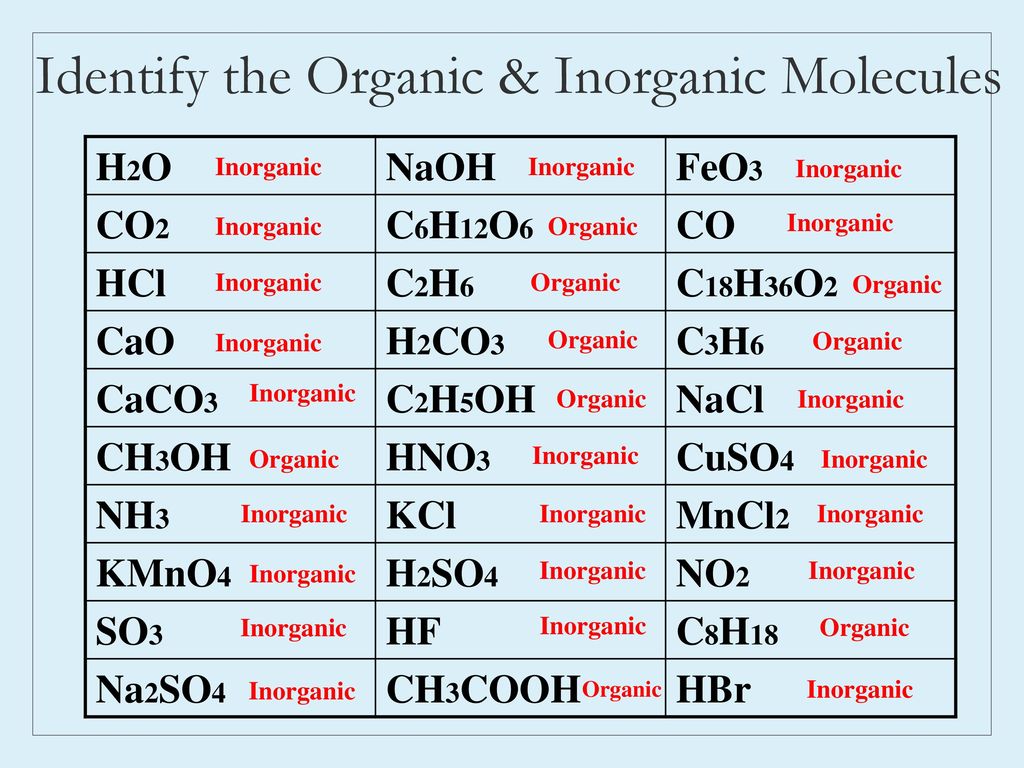
NaOH-extracted inorganic and organic phosphorus (P), as influenced by... | Download Scientific Diagram
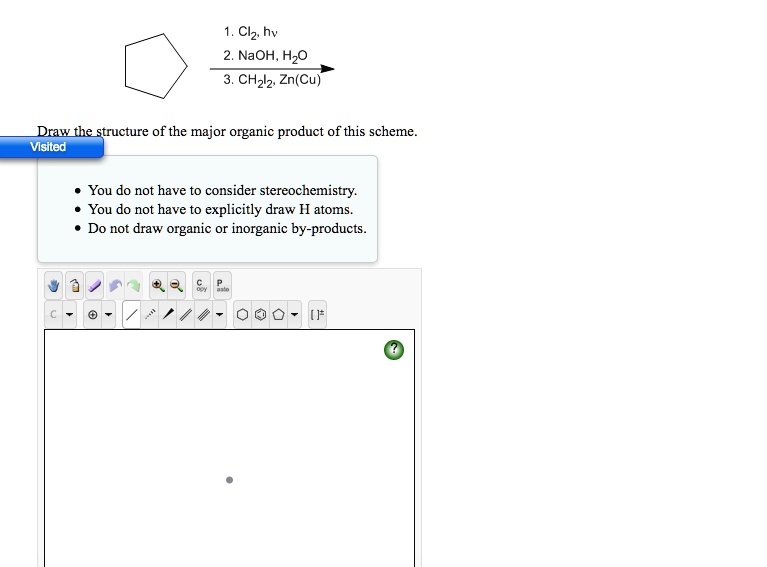
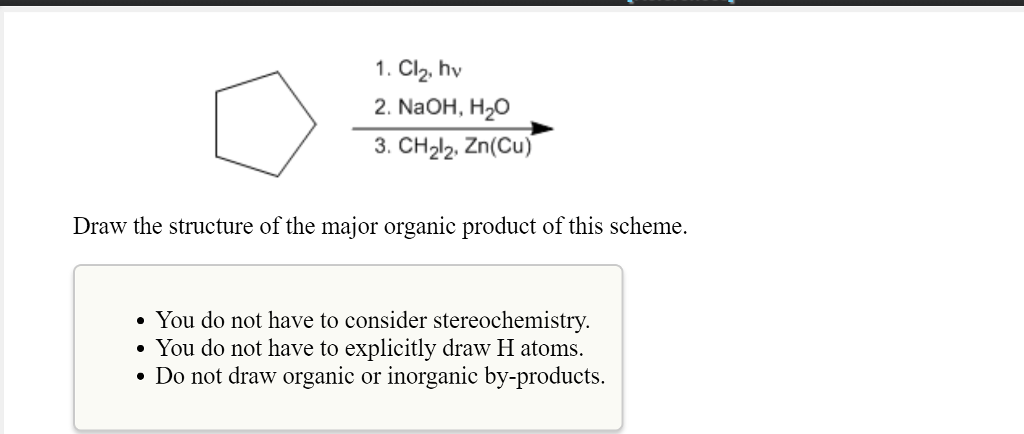
SOLVED: 1. Clz, hv 2. NaOH, H2O 3. CH2Cl2 Zn(Cu) Draw the structure of the major organic product of this scheme. You do not have to consider stereochemistry. You do not have