-Under-the-EU-MDR.png)
Regulation of Custom-made Devices (CMDs) Under the EU MDR | Freyr - Global Regulatory Solutions and Services Company
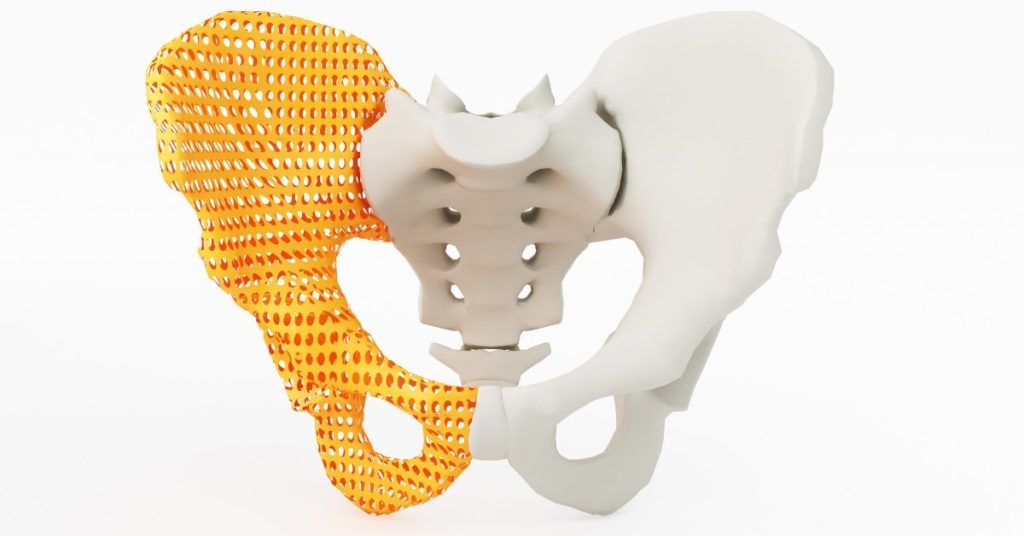
Where Do We Stand with the New EU MDR, Additive Manufacturing, and Customised Medical Devices? - SL Controls

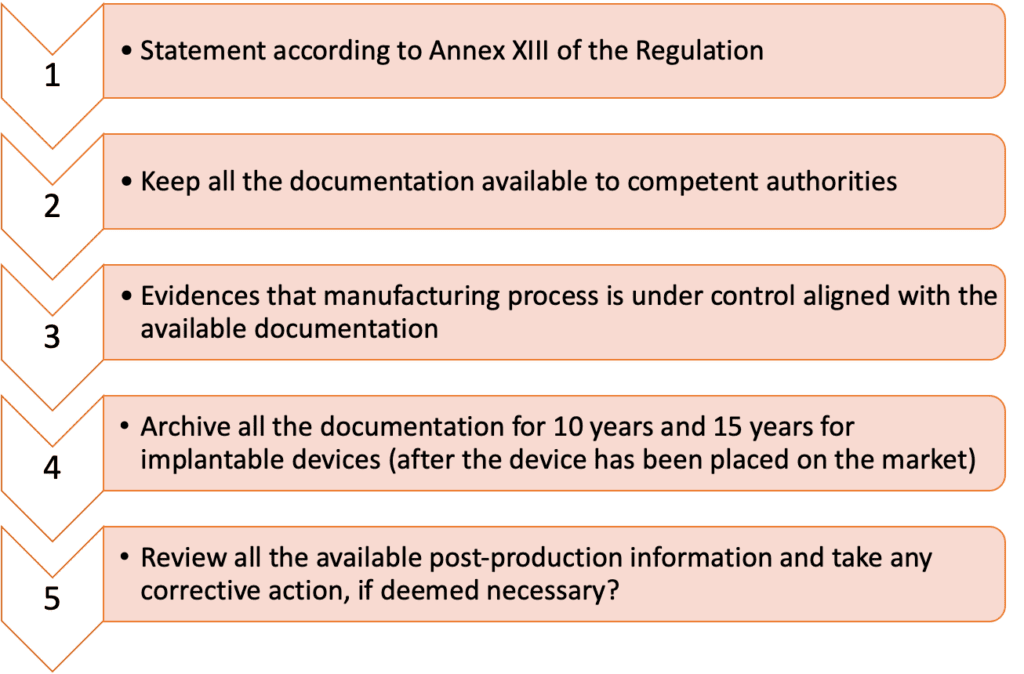
Fusedbone | Definitive Guide to Manufacturing MDR Regulated Custom-made medical devices at the Point of Care

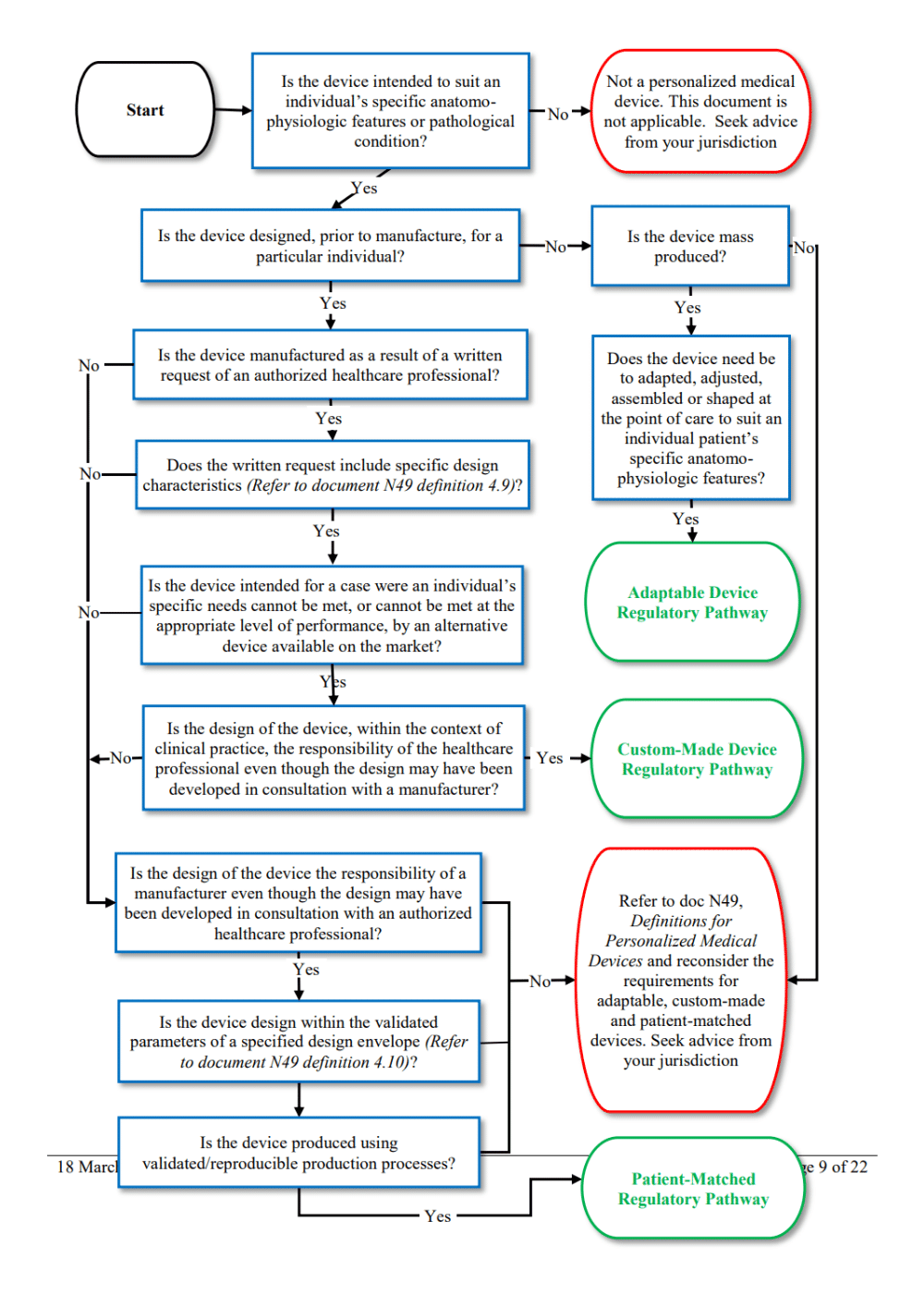
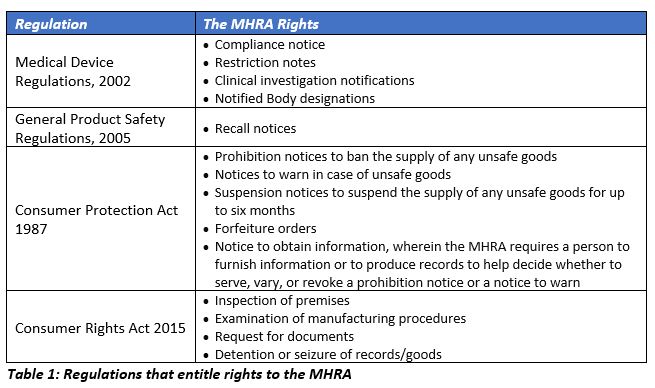
Medical device legislation for custom-made devices after the UK has left the EU: answers to a further ten important questions | British Dental Journal





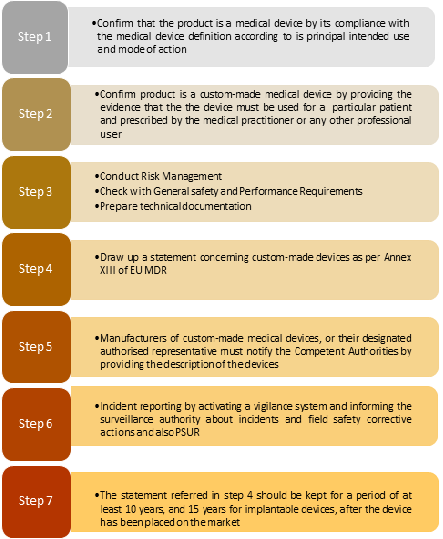



![What are Custom Made devices under EU MDR? [Erik Vollebregt] - YouTube What are Custom Made devices under EU MDR? [Erik Vollebregt] - YouTube](https://i.ytimg.com/vi/GDuBS1bbhyk/hqdefault.jpg)