Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection | Nature Medicine

Evolution of antibody responses up to 13 months after SARS-CoV-2 infection and risk of reinfection - eBioMedicine
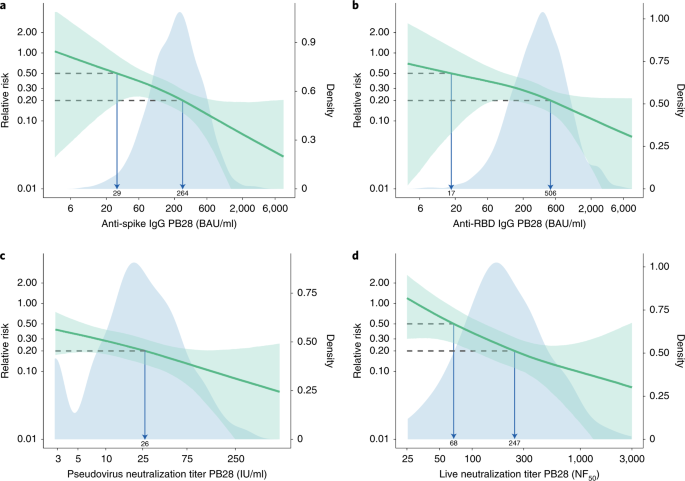
Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection | Nature Medicine

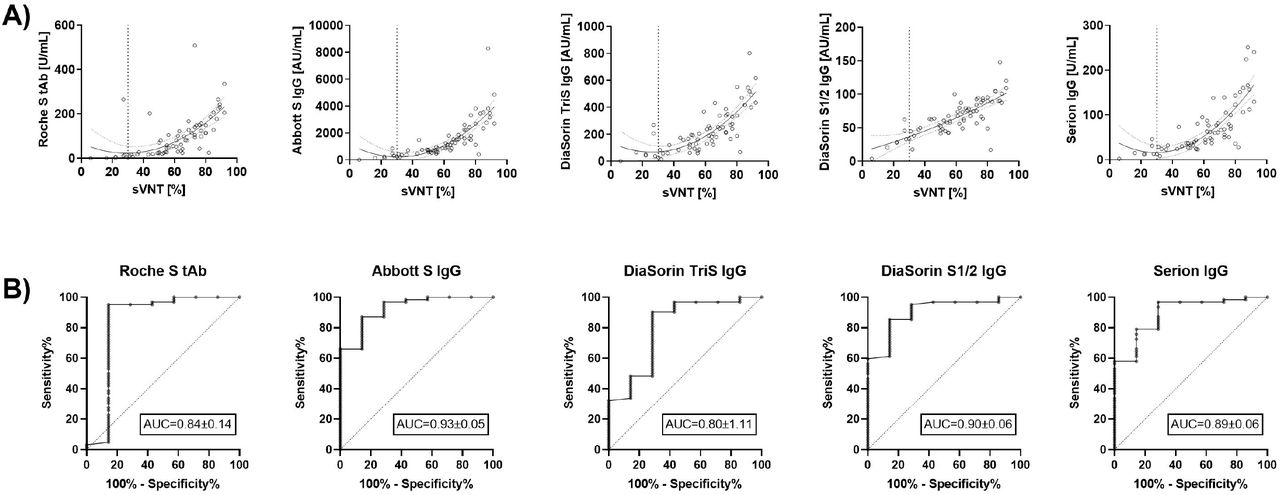
Diagnostics | Free Full-Text | Quantitative SARS-CoV-2 Spike Antibody Response in COVID-19 Patients Using Three Fully Automated Immunoassays and a Surrogate Virus Neutralization Test

Frontiers | The Safety and Immunogenicity of the mRNA-BNT162b2 SARS-CoV-2 Vaccine in Hemodialysis Patients
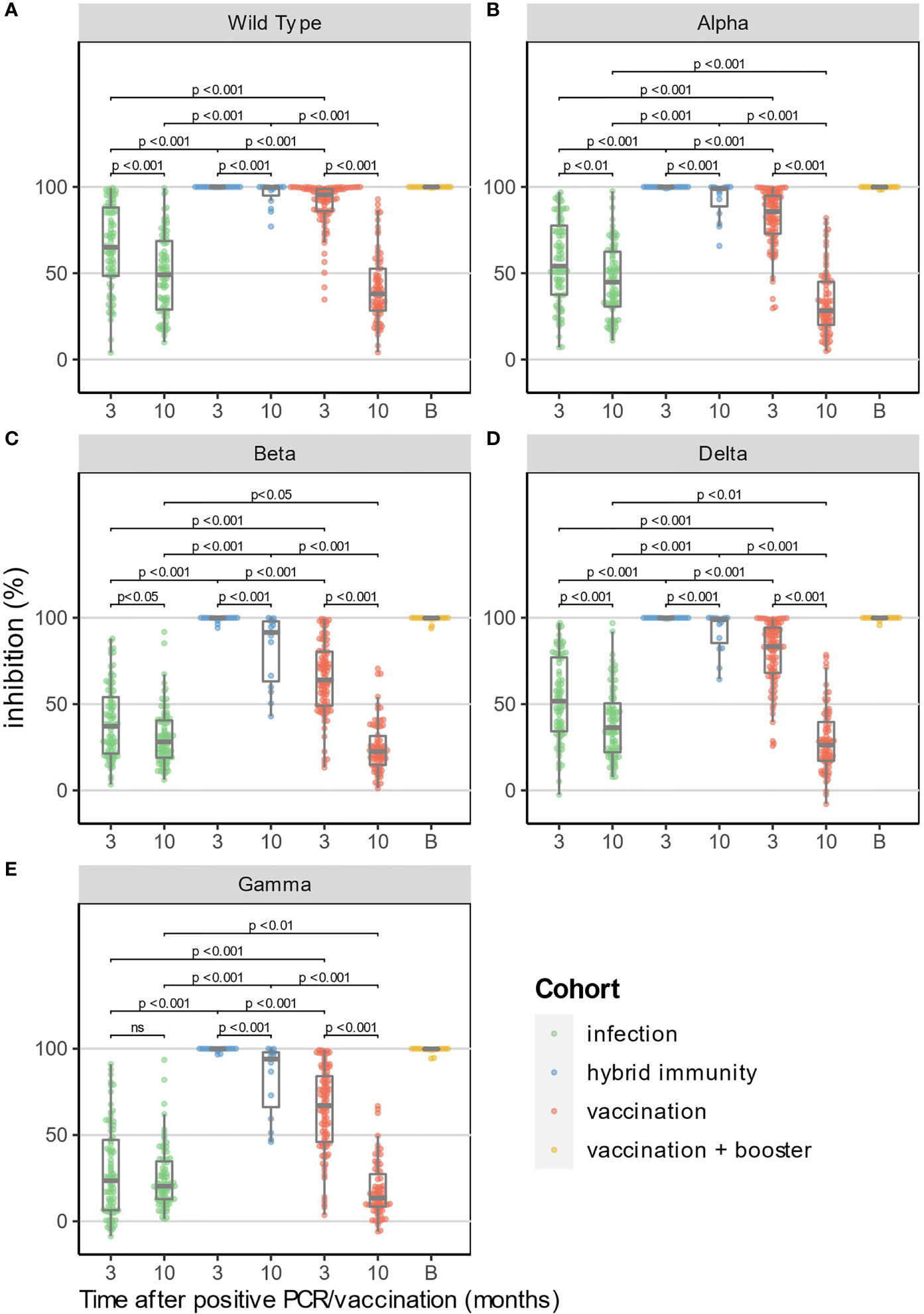
Frontiers | IgG Anti-Spike Antibodies and Surrogate Neutralizing Antibody Levels Decline Faster 3 to 10 Months After BNT162b2 Vaccination Than After SARS-CoV-2 Infection in Healthcare Workers
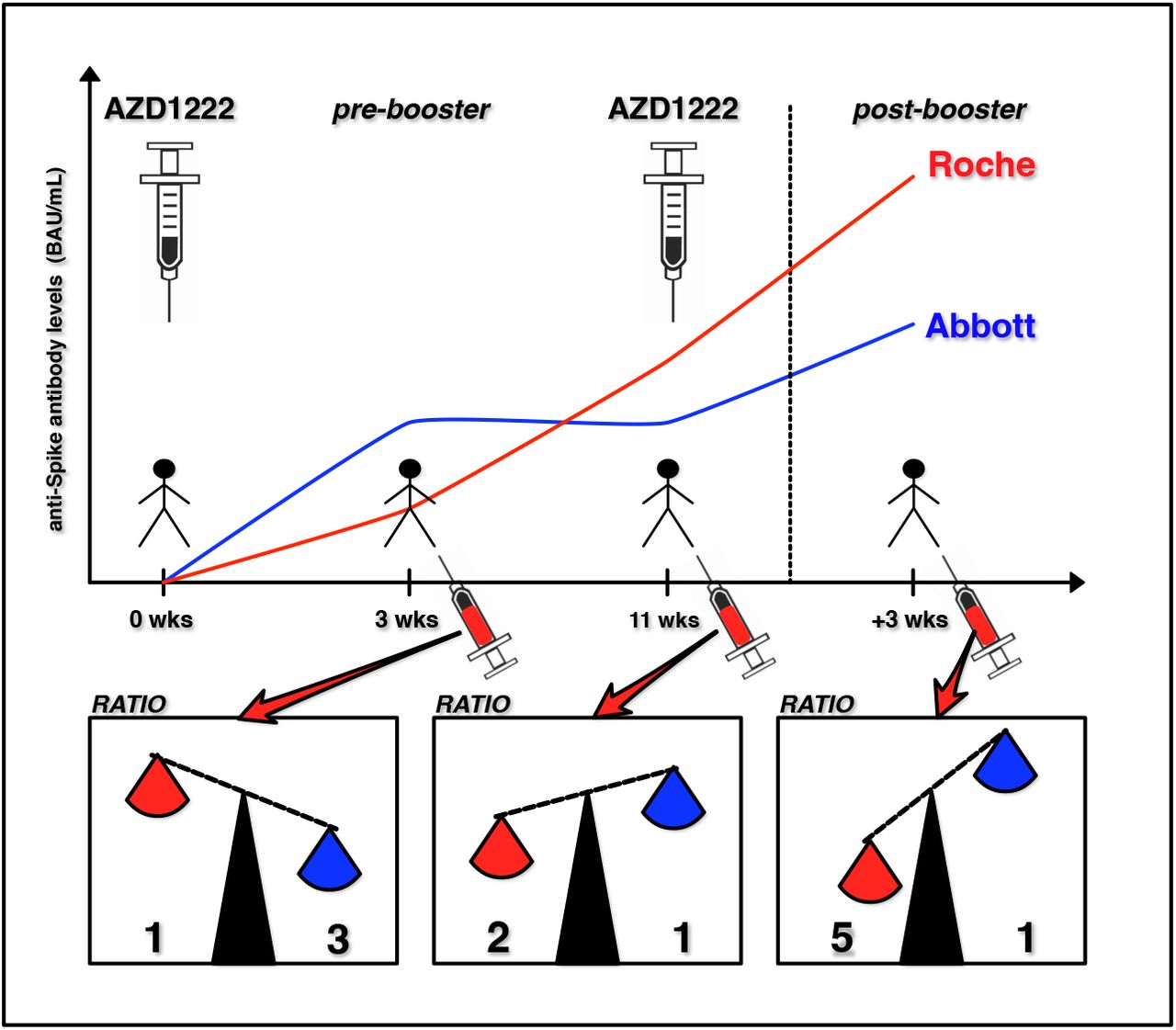
The comparability of Anti-Spike SARS-CoV-2 antibody tests is time-dependent: a prospective observational study | medRxiv
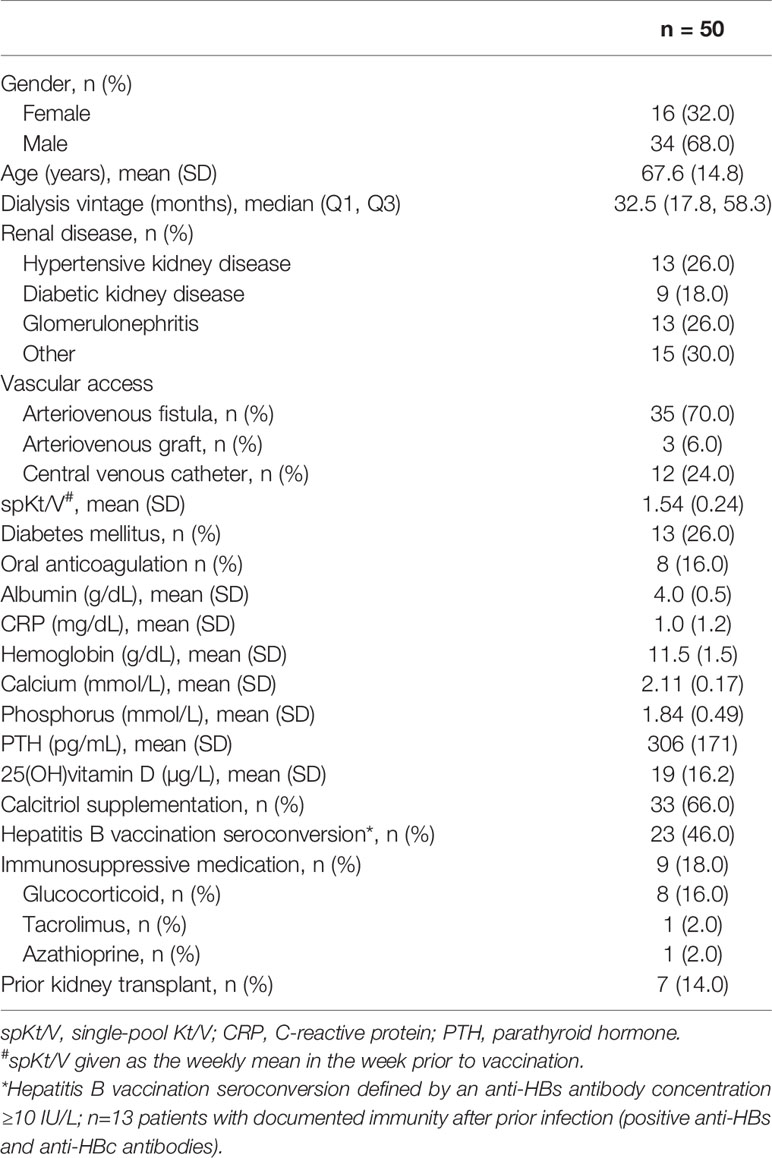
Frontiers | The Safety and Immunogenicity of the mRNA-BNT162b2 SARS-CoV-2 Vaccine in Hemodialysis Patients

Third dose of anti-SARS-CoV-2 vaccine for patients with cancer: Should humoral responses be monitored? A position article - ScienceDirect
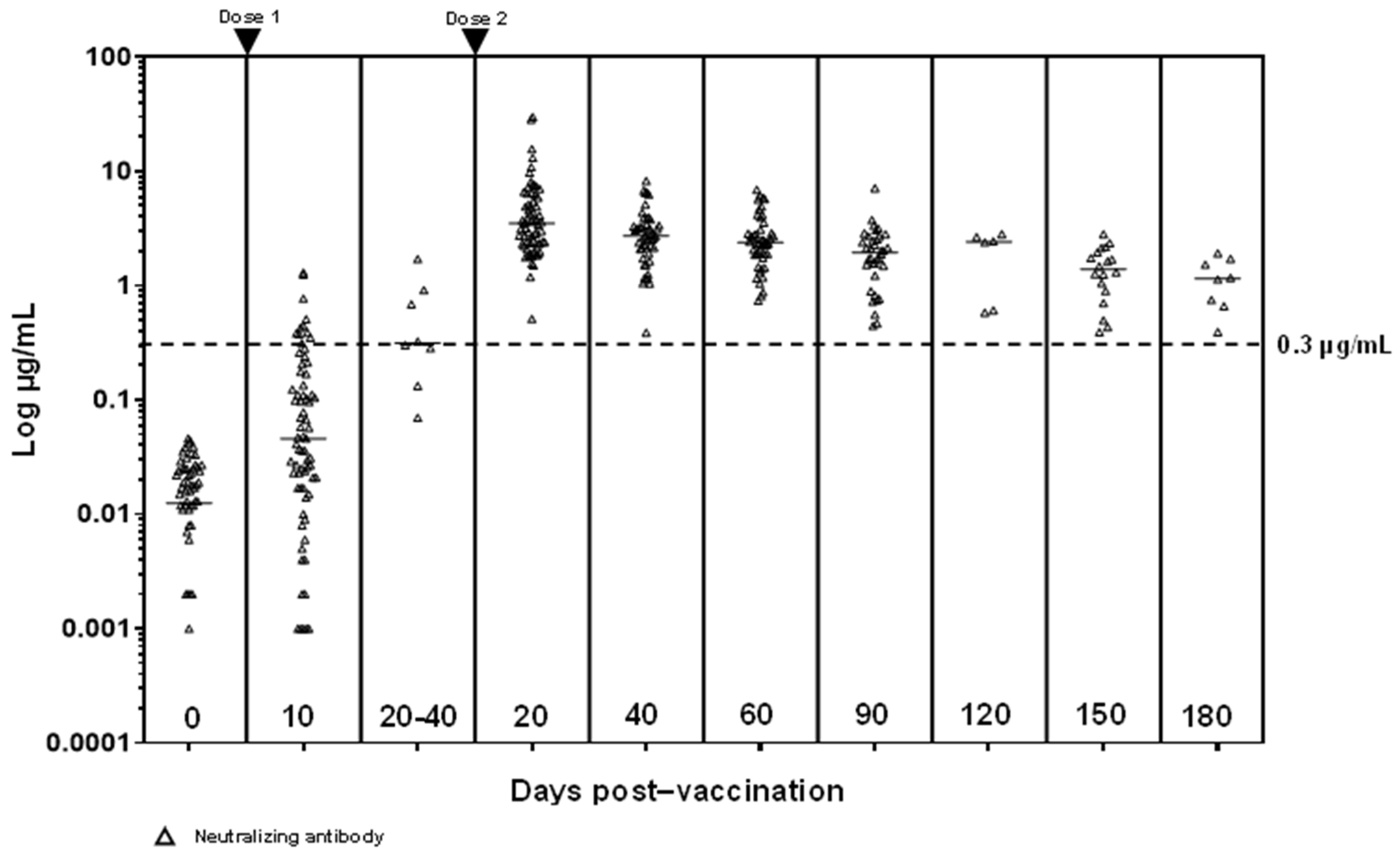
Vaccines | Free Full-Text | Robust SARS-CoV-2 Antibody Responses in Asian COVID-Naïve Subjects 180 Days after Two Doses of BNT162b2 mRNA COVID-19 Vaccine

Evolution of antibody responses up to 13 months after SARS-CoV-2 infection and risk of reinfection - eBioMedicine

Evolution of antibody responses up to 13 months after SARS-CoV-2 infection and risk of reinfection - eBioMedicine
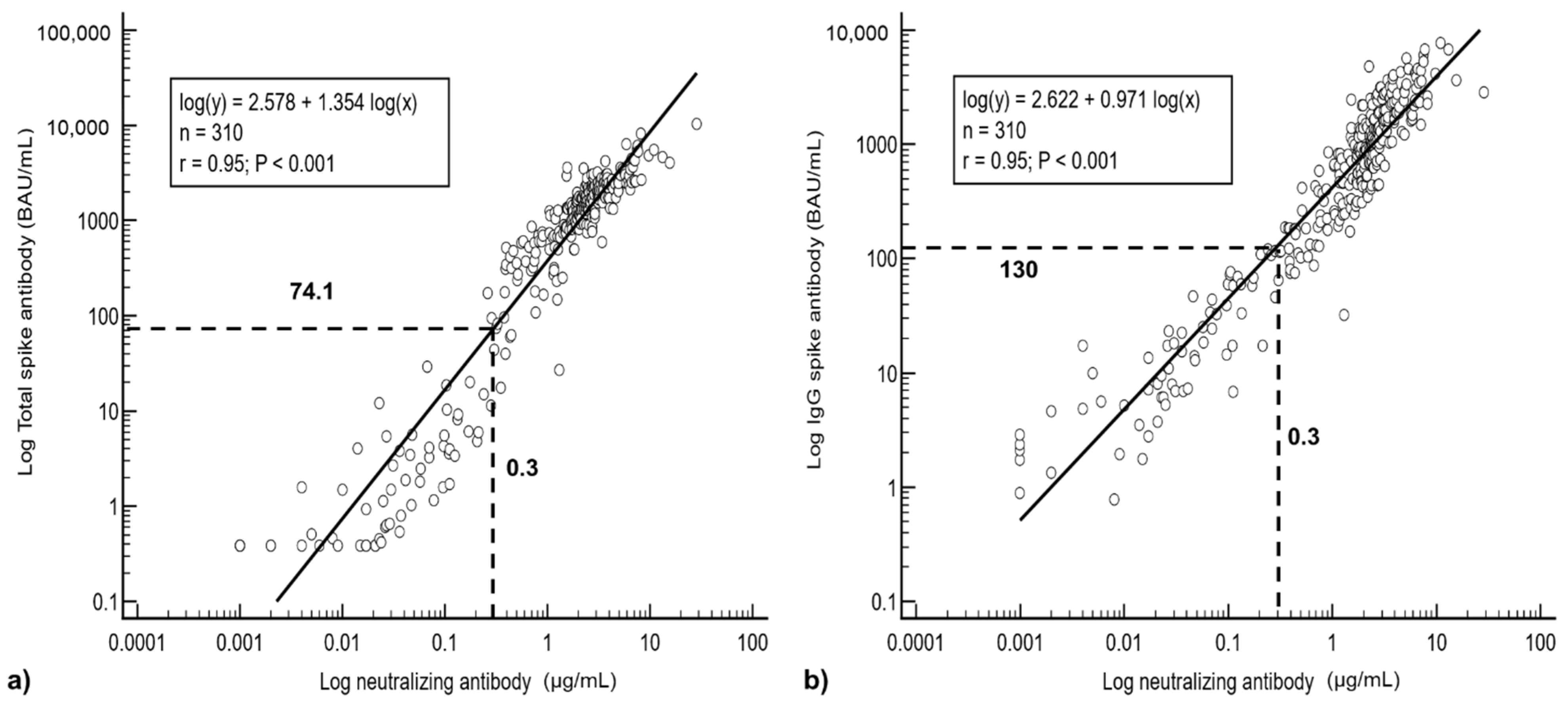
Vaccines | Free Full-Text | Robust SARS-CoV-2 Antibody Responses in Asian COVID-Naïve Subjects 180 Days after Two Doses of BNT162b2 mRNA COVID-19 Vaccine

Third dose of anti-SARS-CoV-2 vaccine for patients with cancer: Should humoral responses be monitored? A position article - ScienceDirect

Evolution of antibody responses up to 13 months after SARS-CoV-2 infection and risk of reinfection - eBioMedicine

Anti-Spike Protein Assays to Determine SARS-CoV-2 Antibody Levels: a Head-to-Head Comparison of Five Quantitative Assays | Microbiology Spectrum

Validation and performance of a multiplex serology assay to quantify antibody responses following SARS‐CoV‐2 infection or vaccination - Wilkins - 2022 - Clinical & Translational Immunology - Wiley Online Library

Validation and performance of a multiplex serology assay to quantify antibody responses following SARS‐CoV‐2 infection or vaccination - Wilkins - 2022 - Clinical & Translational Immunology - Wiley Online Library